Ammonia production is a cornerstone of the chemical industry, with widespread applications in fertilizers, chemicals, and energy storage. The Lummus Ammonia Process, a modern adaptation of the Haber-Bosch process, exemplifies the industry’s drive for efficiency, sustainability, and performance. This article explores the intricate stages of the Lummus process, the critical equipment used, operational parameters, and material considerations.
The Lummus Ammonia Process synthesizes ammonia (NH₃) by reacting nitrogen (N₂) and hydrogen (H₂) under high-pressure and high-temperature conditions. What sets the Lummus process apart is its focus on energy efficiency, process integration, and environmental sustainability.
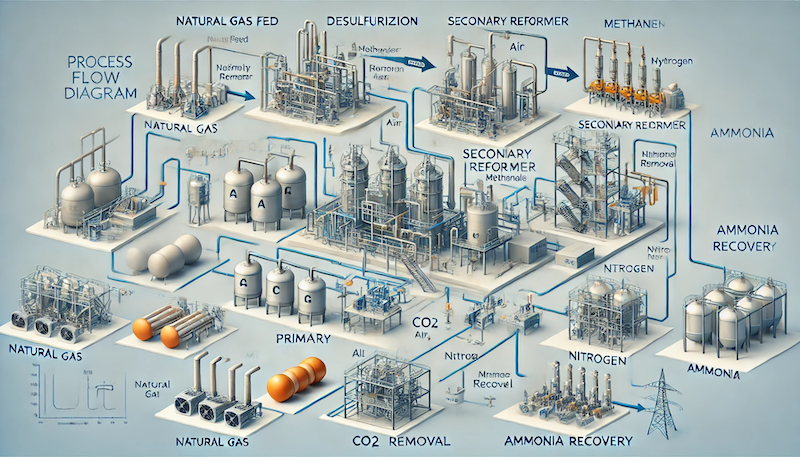
The key stages of the process include:
Energy Efficiency:
Environmental Integration:
Advanced Catalysts:
Material Challenges:
The Lummus Ammonia Process showcases innovation in ammonia production, addressing both industrial demands and environmental concerns. Its integration of advanced catalysts, energy recovery, and sustainable technologies ensures its relevance in the modern chemical industry.