Urea is one of the most important nitrogen-based fertilizers and industrial chemicals in the world. It is synthesized from ammonia (NH₃) and carbon dioxide (CO₂) in a highly efficient and economical process. This guide explores the production of urea in detail, covering process stages, critical equipment, operating conditions, and a process flow diagram (P&ID).
Urea (CO(NH₂)₂) is synthesized through the ammonia stripping process, which leverages the reaction of ammonia and carbon dioxide under high temperature and pressure. The main stages of the process include:
The process is exothermic and produces urea as the final product while recycling unreacted ammonia and carbon dioxide.
Read More: The Lummus Ammonia Process: A Detailed Guide
Energy Efficiency: Heat integration reduces energy consumption, especially in the recycling loop for unreacted NH₃ and CO₂.
Recycling Loop: Up to 85% of unreacted materials are recycled, minimizing raw material waste.
Emission Control: Scrubbers and absorbers limit ammonia emissions and ensure environmental compliance.
Corrosion Resistance: The highly corrosive nature of ammonium carbamate and urea necessitates special materials like duplex and super duplex stainless steel.
Below is a simplified PFD representing the urea production process:
Key Components:
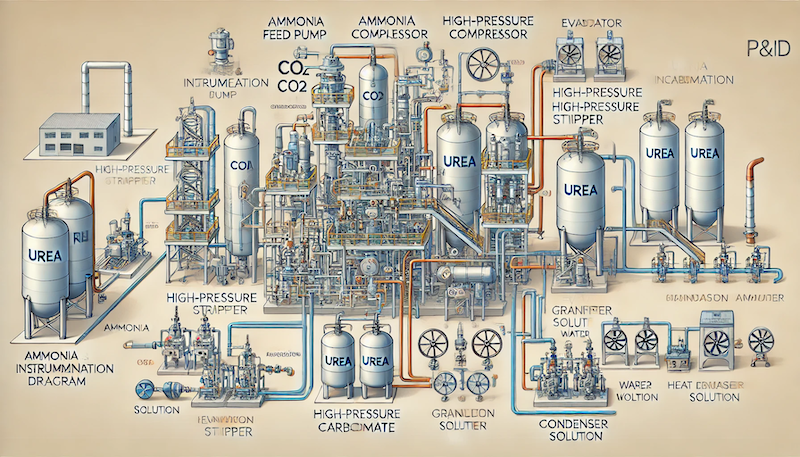
The production of urea from ammonia is a highly optimized and sustainable process. By recycling unreacted materials, integrating heat recovery, and adopting advanced materials, modern urea plants achieve high efficiency and low environmental impact. The use of prilled or granulated urea ensures its safe handling and effective use in agriculture and industry. With continuous advancements in technology, the urea production process continues to evolve, meeting global demands sustainably.